
Paths to Practice Perfection
Simplifying Negative Pressure Wound Therapy
Simplifying Negative Pressure Wound Therapy

There is a revolution occurring with the 3M™ V.A.C.® Negative Pressure Wound Therapy System. 3M™ Dermatac™ Drape, is a silicone-acrylic hybrid drape, that now makes it easier than ever to utilize 3M™ V.A.C.® Therapy. Dermatac Drape can be repositioned upon initial application, and promotes patient comfort at dressing removal. Because of the unique structure of Dermatac Drape, it conforms to different anatomical locations including uneven areas, while still providing a highly effective seal. It can also be repositioned after the initial placement (for up to twenty minutes), instead of having to start over again. This can result in time being saved in dressing application and dressing changes due to its increased ease of use. Because it is so easy to reposition after the initial application, smoothing out any wrinkles that might occur is a breeze, all while maintaining adhesion. Patient comfort is paramount with Dermatac Drape. This speaks to the remarkable properties of Dermatac Drape and its versatility. Patients love the flexibility of this new product. I have had many patients specifically request that only Dermatac Drape be used for their V.A.C.® Therapy Dressings. It also provides excellent skin friendly properties. This is important since much of our healing extends from the peri-wound tissues.1,2,3
Dermatac Drape Product Introduction
A hybrid drape for 3M™ V.A.C.® Therapy: first silicone – acrylic drape that is providing the ideal balance for wound healing support
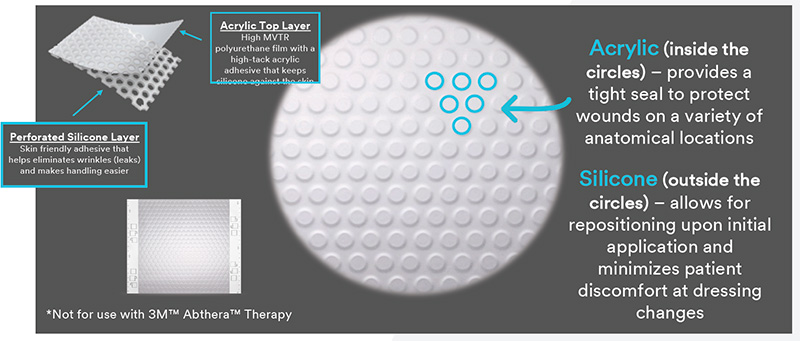
This demonstrates the unique construction of the Dermatac Drape.
What jobs should NPWT drapes do?
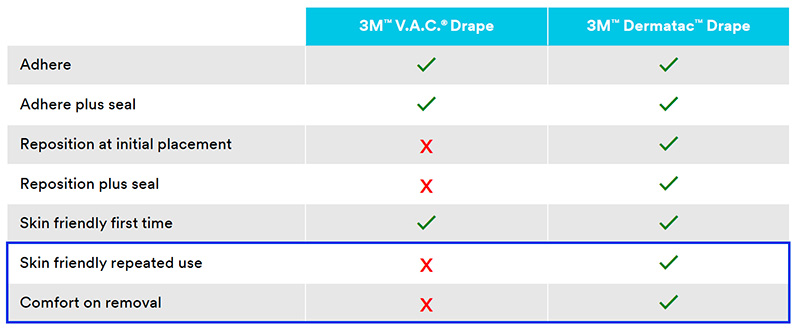
This shows us the differences between the standard V.A.C.® Drape and Dermatac Drape.
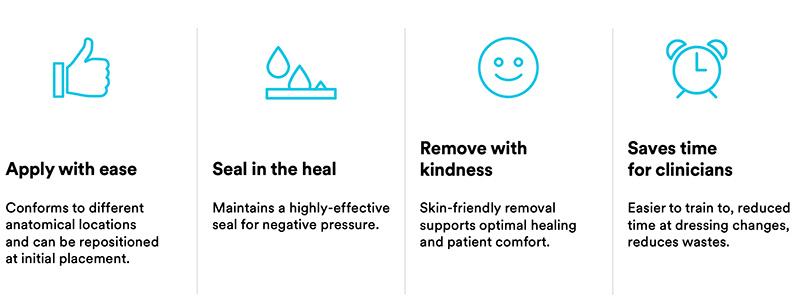
Through innovative dual technology, 3M™ Dermatac™ Drape works hard during each step of 3M™ V.A.C.® Therapy for clinicians and patients
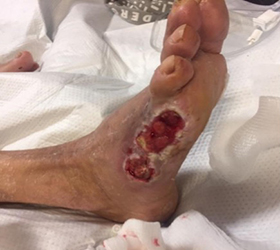
CASE STUDY
A 73-year-old male, with Type II diabetes, diabetic neuropathy, diminished palpable pulses, and an ABI of 0.8, and toe brachial index of 0.28. He is post 5th ray resection of his right foot after diabetic ulceration and osteomyelitis. He is now two months post-surgical treatment and has been readmitted to the hospital for an angiogram to determine his vascular status. He is deemed not a revascular candidate and negative pressure wound therapy is recommended. He has a stalled wound with little change in wound dimensions. His peri-wound skin is very fragile, and it was determined that he might benefit from a different V.A.C.® drape. Dermatac Drape was then used for this patient.
- S/P 5th ray resection
- ABI of 0.8
- Brachial index of 0.28
- Wound has been unable to close for two months
- Fragile peri-wound skin
Co-morbidities
- Type II Diabetic
- Diabetic Neuropathy
Wound Progression
Presentation at the introduction of Dermatac Drape
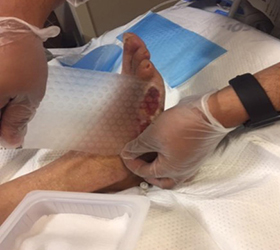
Protection of peri-wound skin and application of Dermatac Drape and V.A.C.®Granufoam™ Dressing


A 15% total decrease in wound volume after just two days of V.A.C.® Therapy and Dermatac Drape use and a marked decrease in peri-wound tissue maceration.
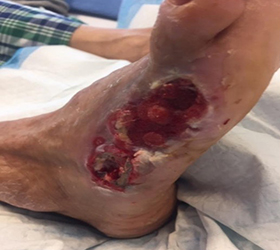
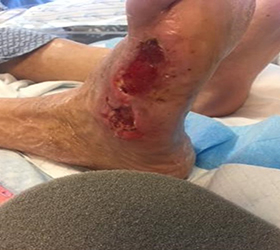
The wound showed significant improvement after two dressing changes while using the Dermatac Drape and V.A.C.® Therapy. After the second change, excellent wound edge viability and the wound showed great progression towards wound closure.
Conclusion
The case presentation above showed the use of V.A.C.® Therapy with the silicone-acrylic hybrid drape, Dermatac Drape, which resulted in very good wound progression in a short period of time, with ease of use and a decrease in peri-wound tissue maceration.
Note: Dr. Garoufalis is a paid consultant for 3M products. Patient data and photos courtesy of Matthew G. Garoufalis DPM, FASPS, FACPM, CWS, FFPM RCPS (Glasg).
As with any case study, the results and outcomes should not be interpreted as a guarantee or warranty of similar results. Individual results may vary depending on the patient’s circumstances and condition.
NOTE: Specific indications, contraindications, warnings, precautions, and safety information exist for these products and therapies. Please consult a clinician and product instructions for use prior to application. Rx only.
References
- Moues CM, Vos MC, van den Bemd GJ, Stijnen T, Hovius SER. Bacterial load in relation to vacuum–assisted closure wound therapy: a prospective randomized trial. Wound Repair Regen. Jan-Feb 2004;12(1):11-17.
- de Laat EH, van den Boogaard MH, Spauwen PH, van Kuppevelt DH, van Goor H, Schoonhoven L. Faster wound healing with topical negative pressure therapy in difficult-to-heal wounds: a prospective randomized controlled trial. Ann Plast Surg. 2011 Dec 1;67(6):626-631.
- 3. Yao M, Fabbi M, Hayashi H, Park N, Attala K, Gu G, French MA, Driver VR. A retrospective cohort study evaluating efficacy in high-risk patients with chronic lower extremity ulcers treated with negative pressure wound therapy. Int Wound J. 2014 Oct;111(5):483-488. Epub 2012 Nov 19.
Do you have questions regarding this case study?
Connect with a 3M Account Representative to learn more.
© 2022 3M. All rights reserved. 3M and the other marks shown are marks and/or registered marks. Unauthorized use prohibited.


Comments
There are 0 comments for this article